PACE Biology
PACE Elicits Biologic Effects
Pulsed Acoustic Cellular Expression (PACE) technology utilizes high-energy acoustic pressure waves in the shock wave spectrum to produce compressive and tensile stresses on cells and tissue structures to elicit a series of biological responses. PACE treatment results in an increase in perfusion and arteriogenesis, biofilm disruption, a pro-inflammatory response, cytokine and chemokine effects, growth factor upregulation, angiogenesis (new blood vessel formation) and the subsequent regeneration of tissue such as skin, musculoskeletal and vascular structures.
Mechanism of Action
The biological responses to shock wave treatment have been studied for over 30 years, although some of the most clinically relevant developments regarding tissue regeneration are recent discoveries. The peer-reviewed scientific literature regarding medical shock wave treatment is well documented and extensive. The results, individually and collectively, present compelling evidence that PACE may be benefical in the body's healing process. SANUWAVE makes no claim to safety and effectiveness, and the following information is presented for educational purposes only.
Perfusion and Arteriogenesis
PACE treatment leads to an increase in perfusion. As the PACE shock waves penetrate the microcirculatory system, there is an immediate change in local blood flow in the treated area. Li et al determined that local blood perfusion increased as early as 2 to 8 hours after treatment due to the vasodilation (increasing diameter) of pre-existing vessels [1]. Research performed at Cleveland Clinic using Doppler readings to measure blood flow in treatment areas showed an increase in blood perfusion and vessel density 24 hours after treatment [2]. This increase in perfusion is important because it decreases the ischemia (lack of blood flow) that is often associated with impaired healing. Patients receiving treatment did not experience any unsafe change in blood pressure due to the increased perfusion [3].
These Doppler images indicate more red areas of improved circulation after PACE treatment of this deep partial/full thickness burn.
Biofilm
Antibiotic-resistant bacterial colonies often produce biofilms. A biofilm is a defensive mechanism that creates a physical protective barrier against antibiotic treatment. Wanner et al concluded that shock wave treatment can break up the physical biofilm barriers and allow antibiotics to gain access to the entrenched bacteria and eradicate the colony [4].
Inflammatory Response
An immediate inflammatory response is apparent after PACE treatment. Researchers at Cleveland Clinic have reported a trend in decreasing rolling and sticking leukocytes (white blood cells), and an increase in transmigrating leukocytes moving through the vessel wall and into treatment area tissues in response to PACE treatment [5].
Increasing leukocyte activation assists in the inflammatory phase of wound healing by triggering the release of pro-angiogenic factors. After shock wave treatment, wounds move quickly through the inflammatory phase to the proliferation (cell duplication) phase of healing [6,7].
Cytokines and Chemokines
Studies show that the early pro-angiogenic and pro-inflammatory responses to PACE treatment are accompanied by significantly increased expression of both CD31 and angiogenesis pathway-specific genes, including ELR-CXC chemokines (CXCL1, CXCL2, CXCL5), CC chemokines (CCL2, CCL3, CCL4), cytokines (IL-1B, IL-6, G-CSF, VEGF-A), matrix metalloproteinases (MMP3, MMP9, MMP13), hypoxia-inducible factors (HIF-1a), and vascular remodeling kinase (Mst1), as early as 6 hours and up to 7 days post-treatment [2,6,7]. This may be evidence of an immediate and long-term angiogenic and pro-inflammatory healing response.
Further, PACE treatment significantly decreased neutrophil and macrophage (white blood cell) infiltration into the wound, attenuating both CC- and CXC-chemokines at the wound margin [6]. This may indicate a change from a chronic, non-healing wound to an acute healing state.
Apoptosis is the rate at which cells are replaced by the body, and an increased rate of apoptosis can be associated with disease states. However, shock wave treatment was found to decrease the rate of apoptosis in disease states to normal levels [8]. Wang et al reported a statistically significant decrease in TUNEL (indicator of apoptosis) after PACE treatment [8].
Growth Factor Upregulation
At a cellular level, PACE treatment applies mechanical forces to individual cells in the treated area. The cells have a biological response to these mechanical forces, referred to as cellular expression. Cellular expression occurs when the genes in the cell are activated to produce wound healing proteins known as growth factors. Pro-angiogenic growth factors such as eNOS, VEGF, vWF, PCNA, EGF and others are upregulated as a result of shock wave treatment [2,6,7,8]. These factors start a cascade of cellular activities that cause an increase in cellular proliferation and tissue regeneration. Growth factor upregulation has been shown to persist for up to 12 weeks [9].
Angiogenesis
The pro-angiogenic factors released in response to PACE treatment lead to new vessel formation resulting in the creation of new capillary networks in the treated area. Vascular endothelial growth factor (VEGF) corresponds to the growth of new blood vessels that allow blood flow improvement in a wound area. Wang et al reported an increase in VEGF after PACE treatment [8]. Davis et al reported that by day 7, shock wave treatment created a greater number of blood vessels versus untreated controls [7]. Another series of studies compared the effects of shock wave treatment with a direct gene therapy application of VEGF in ischemic tissue [10,11,12]. The shock wave treatment actually outperformed direct topical VEGF applications in these studies.
Granulation
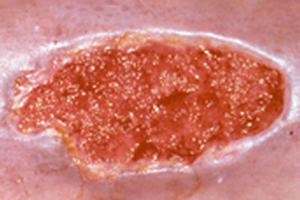
Cellular proliferation is one of the most noticeable stages of wound healing. During this stage, cells divide and cover the wound surface to close the wound. This process begins with a granulation tissue phase that builds vascularized tissue in the wound defect. Proliferating cell nuclear antigen (PCNA) is a growth factor indicating that this stage of wound healing is progressing. Wang et al reported a statistically significant increase in average PCNA levels after PACE treatment [8]. This finding indicates that PACE treatment may accelerate wound granulation. Stojadinovic et al reported marked granulation tissue development on post-treatment day 4 [7]. Saggini et al reported that the percent of granulation tissue increased significantly in the wounds of patients treated with shock wave after treatment [13].
Epithelialization
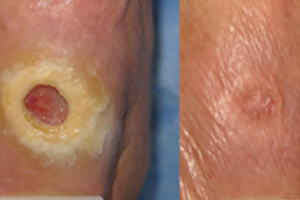
Results of a recent Phase III clinical trial strongly suggest that dermaPACE has an effect in the stabilization, size reduction and, with time, complete re-epithelialization of wounds, specifically diabetic foot ulcers. Clinically significant re-epithelialization of greater than 90% was demonstrated to have statistical significance at 12 weeks in favor of PACE treated wounds (51/107, 47.7%) compared with Sham-control wounds (31/99, 31%) (p=0.016). Furthermore, of the wounds that achieved at least 90% wound area reduction at 12 weeks, the median reduction in area exceeded 99%. Overall, PACE treated wounds were twice as likely to achieve 90-100% wound closure compared with Sham-control subjects within 12 weeks of the initial PACE procedure. Further, by 12 weeks, the reduction in target ulcer area in PACE subjects was on average 48.6% compared with an average of only 10.7% in subjects randomized to Sham-control (p=0.015) [14].
References
- Li et al, Improvement of Blood Flow, Expression of Nitric Oxide, and Vascular Endothelial Growth Factor by Low-Energy Shockwave Therapy in Random-Pattern Skin Flap Model. Annals of Plastic Surgery. 2008 Dec;61(6):646-53.
- Krokowicz et al., Microcirculatory response to shockwave therapy in acute model - preliminary report. Presented during the International Society for Musculoskeletal Shockwave Therapy, Toronto, Canada, June 2007.
- Sanctis et al. Effects of Shock Waves on the Microcirculation in Critical Limb Ischemia (CLI) (8-Week Study). Angiology. 2000 Aug;51(8:2): S69-78.
- Wanner et al. Low-energy shock waves enhance the susceptibility of staphylococcal biofilms to antimicrobial agents in vitro. J Bone Joint Surg Br. 2011 Jun;93(6):824-7.
- Siemionow et al. Pulsed Acoustic Cellular therapy Supports Pro-angiogenic Factors Expression in Ischemic Muscles. Poster presentation at the Diabetic Foot Conference 2008.
- Davis et al. Extracorporeal Shock Wave Therapy Suppresses the Early Proinflmamatory Immune Response to a Severe Cutaneous Burn Injury. International Wound Journal. Vol 6, No 1. 2008.
- Stojadinovic et al. Angiogenic response to Extracorporeal Shock Wave Treatment in Murine Skin Isografts. Angiogenesis. 2009 2008;11(4):369-80
- Wang et al., Molecular Changes in Diabetic Foot Ulcers. Diabetes Research and Clinical Practice. 2011.
- Wang CJ et al., Biological Mechanism of Musculoskeletal Shockwaves. International Society for Musculoskeletal Shockwave Therapy Newsletter, Volume 1, Issue 1, 2004.
- Meirer R, Brunner A, Deibl M, Oehlbauer M, Piza-Katzer H, Kamelger FS, Shock wave therapy reduces necrotic flap zones and induces VEGF expression in animal epigastric skin flap model. J Reconstr Microsurg. 2007 May; 23(4):231-6.
- Meirer R, Heumer GM, Oehlbauer M, Wanner S, Piza-Katzer H, Kamelger FS, Comparison of the effectiveness of gene therapy with vascular endothelial growth factor or shockwave therapy to reduce ischemic necrosis in an epigastric skin flap model in rats. J Plast Reconstr Aesthet Surg. 2007;60(3):266-71.
- Kamelger et al. Comparison of the Effectiveness of Gene Therapy with Vascular Endothelial Growth Factor or Shock Wave Therapy to Reduce Ischaemic Necrosis in an Epigastric Skin Flap Model in Rats. 2007; 60:266-271.
- Saggini et al. Extracorporeal shock wave therapy for management of chronic ulcers in the lower extremities. Ultrasound Med Biol. 2008 Aug;34(8):1261-71.
- Phase III Pivotal Trial Results of dermaPACE for the Treatment of Diabetic Foot Ulcers. Data on file with SANUWAVE Health, Inc.
